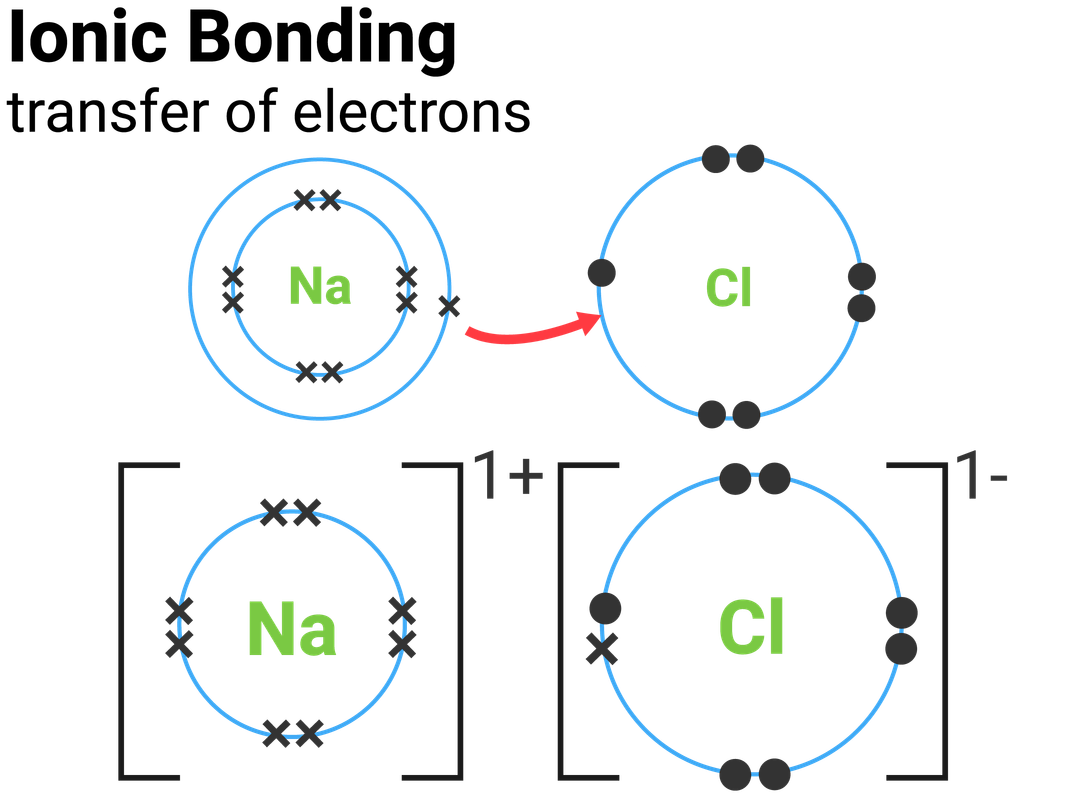
Ionic Bonds Form Between Metals And . Ionic and covalent bonds differ in the degree of the sharing of the electron density between the atoms involved in the. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. learn what an ionic bond is, how it forms between metals and nonmetals, and what properties it has. A metal (which forms the. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. the formation of ionic compounds. See examples of ionic compounds and how to predict ionic bonding using electronegativity. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Binary ionic compounds are composed of just two elements: Learn more about ionic bonds in this article. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged.
from revisechemistry.uk
Learn more about ionic bonds in this article. A metal (which forms the. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. the formation of ionic compounds. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. See examples of ionic compounds and how to predict ionic bonding using electronegativity. Binary ionic compounds are composed of just two elements: learn what an ionic bond is, how it forms between metals and nonmetals, and what properties it has.
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk
Ionic Bonds Form Between Metals And Learn more about ionic bonds in this article. Binary ionic compounds are composed of just two elements: Ionic and covalent bonds differ in the degree of the sharing of the electron density between the atoms involved in the. learn what an ionic bond is, how it forms between metals and nonmetals, and what properties it has. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. the formation of ionic compounds. A metal (which forms the. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Learn more about ionic bonds in this article. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. See examples of ionic compounds and how to predict ionic bonding using electronegativity.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Ionic Bonds Form Between Metals And compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Ionic and covalent bonds differ in the degree of the sharing of the electron density between the atoms involved in the. Binary. Ionic Bonds Form Between Metals And.
From slidetodoc.com
Ionic Bonds Chapter 8 Section 2 Ionization Energy Ionic Bonds Form Between Metals And Binary ionic compounds are composed of just two elements: learn what an ionic bond is, how it forms between metals and nonmetals, and what properties it has. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. the formation of ionic compounds. Learn more about ionic bonds in this. Ionic Bonds Form Between Metals And.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds Ionic Bonds Form Between Metals And By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Binary ionic compounds are composed of just two elements: Learn more about ionic bonds in this article. See examples of ionic compounds and how to predict ionic bonding using electronegativity. Such a bond forms when the valence (outermost) electrons. Ionic Bonds Form Between Metals And.
From quizlet.com
5 ) Ionic Bonding Diagram Quizlet Ionic Bonds Form Between Metals And Ionic and covalent bonds differ in the degree of the sharing of the electron density between the atoms involved in the. the formation of ionic compounds. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. learn what an ionic bond is, how it forms between metals and nonmetals, and. Ionic Bonds Form Between Metals And.
From www.vedantu.com
Exploring the Key Difference between ionic covalent and metallic bonds Ionic Bonds Form Between Metals And Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Learn more about ionic bonds in this article. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. See examples of ionic compounds and how to predict ionic bonding using electronegativity. compounds. Ionic Bonds Form Between Metals And.
From www.expii.com
Ionic Bond — Formation & Compounds Expii Ionic Bonds Form Between Metals And ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. A metal (which forms the. the formation of ionic compounds. Binary ionic compounds are composed of just two elements:. Ionic Bonds Form Between Metals And.
From www.thoughtco.com
Examples of Ionic Bonds and Ionic Compounds Ionic Bonds Form Between Metals And By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Ionic and covalent bonds differ in the degree of the sharing of the electron density between the atoms involved in the. the formation of ionic compounds. Binary ionic compounds are composed of just two elements: learn what. Ionic Bonds Form Between Metals And.
From sciencenotes.org
Ionic Bond Definition and Examples Ionic Bonds Form Between Metals And See examples of ionic compounds and how to predict ionic bonding using electronegativity. Binary ionic compounds are composed of just two elements: Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Ionic and covalent bonds differ in the degree of the sharing of the electron density between the atoms involved in. Ionic Bonds Form Between Metals And.
From slideplayer.com
Overview Bonding, Structure and the properties of matter ppt download Ionic Bonds Form Between Metals And A metal (which forms the. the formation of ionic compounds. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Binary ionic compounds are composed of just two elements: See examples of ionic compounds and how to predict ionic bonding using electronegativity. Ionic and covalent bonds differ in. Ionic Bonds Form Between Metals And.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID2702021 Ionic Bonds Form Between Metals And See examples of ionic compounds and how to predict ionic bonding using electronegativity. the formation of ionic compounds. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged.. Ionic Bonds Form Between Metals And.
From www.breakingatom.com
Ionic Bonding Ionic Bonds Form Between Metals And See examples of ionic compounds and how to predict ionic bonding using electronegativity. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. Learn more about ionic bonds in this article.. Ionic Bonds Form Between Metals And.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Ionic Bonds Form Between Metals And Binary ionic compounds are composed of just two elements: A metal (which forms the. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. See examples of ionic compounds and how to predict ionic bonding using electronegativity. Such a bond forms when the valence (outermost) electrons of one atom. Ionic Bonds Form Between Metals And.
From www.slideserve.com
PPT Chemical Bonds PowerPoint Presentation, free download ID3450339 Ionic Bonds Form Between Metals And A metal (which forms the. Learn more about ionic bonds in this article. See examples of ionic compounds and how to predict ionic bonding using electronegativity. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. By definition, a metal is relatively stable if it loses electrons to form a. Ionic Bonds Form Between Metals And.
From slidetodoc.com
Overview Bonding Bonding Chemical bonds Ionic bonding Ionic Ionic Bonds Form Between Metals And Ionic and covalent bonds differ in the degree of the sharing of the electron density between the atoms involved in the. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. A metal. Ionic Bonds Form Between Metals And.
From www2.victoriacollege.edu
formation of ionic bonds Ionic Bonds Form Between Metals And Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Ionic and covalent bonds differ in the degree of the sharing of the electron density between the atoms involved in the. learn what an ionic bond is, how it forms between metals and nonmetals, and what properties it has. Learn more. Ionic Bonds Form Between Metals And.
From en.wikipedia.org
Ionic bonding Wikipedia Ionic Bonds Form Between Metals And compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. Ionic and covalent bonds differ in the degree of the sharing of the electron density between the atoms involved in the. the formation of ionic compounds. Such a bond forms when the valence (outermost) electrons of one atom are. Ionic Bonds Form Between Metals And.
From www.youtube.com
Ionic bonds Reaction of metals & Nonmetals Metals and non metals Ionic Bonds Form Between Metals And Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. the formation of ionic compounds. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held. Ionic Bonds Form Between Metals And.
From www.revisechemistry.uk
Bonding and Properties of materials OCR Gateway C2 revisechemistry.uk Ionic Bonds Form Between Metals And A metal (which forms the. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Learn more about ionic bonds in this article. ionic bond, type of linkage formed from the. Ionic Bonds Form Between Metals And.